CUSTOMIZED COLLECTION
Select groups |
|---|
| Serum | Plasma » |
| PCR | Viral serum matrix » |
| R&D | assay validation » |
| Pre-clinical specimen | Patient samples » |
serum | plasma
Negative Plasma & Serum
All materials are freshly drawn on customer request, and are produced at US FDA registered and approved blood banks and are fully virus tested according to the regulation with FDA approved test procedures or in accordance with European directives.
Human Plasma |
||
|---|---|---|
| Code | Specificity | Form |
| NP0130 | anti-HBs neg. source | single |
| NP0210 | anti-HBs neg. source, customized | single |
| NP0230 | anti-HBs & anti-HBc neg source | single |
| NP1201 | anti-HTLV I/II negative source | single |
| NP1230 | anti-HBs & anti-HBc & anti-HTLV- I/II neg source | single |
Custom Anticoagulants |
||
|---|---|---|
| Code | Specificity | Form |
| NP0110 | ACDA Plasma | single / pooled |
| NP0200 | Human Plasma, Citrate | single / pooled |
| NP0300 | Normal Plasma CPD | single / pooled |
| NP0990 | Normal Plasma, Lithium Heparin | single / pooled |
| NP0992 | Normal Plasma, K3 EDTA | single / pooled |
| NP0993 | Normal Plasma, Na2 EDTA | single / pooled |
| NP0995 | Normal Plasma, Sodium Heparin | single / pooled |
Human Whole Blood & Blood Components |
||
|---|---|---|
| Code | Specificity | Form |
| SN0001 | Whole Blood | single |
| SN0010 | Erythrocyte Concentrate | single / pooled |
| NP0020 | Red Blood Cells (Na2 EDTA) | single / pooled |
| SN0050 | Buffy Coats | single |
| SN0060 | SAG-M red Cells Concentrate | liquid |
| SN0992 | K3 EDTA Whole Blood | frozen |
PCR | viral serum matrix
Viral positive Bulk0.5 – 200 ml, serum and plasma fully tested and characterized |
||||
|---|---|---|---|---|
| Marker | Viral load | Genotype | Serology | Sequence / Mutation |
| WNV | • | • | ||
| HIV | • | • | ||
| HCV | • | • | • | • |
| HBV | • | • | • | • |
| Parvo B19 | • | |||
| CMV | • | • | • | |
| HAV | • | • | • | |
For each marker we also manufacture negative and control material.
Genotyped Plasma / Serum
R&D | validations & characterization
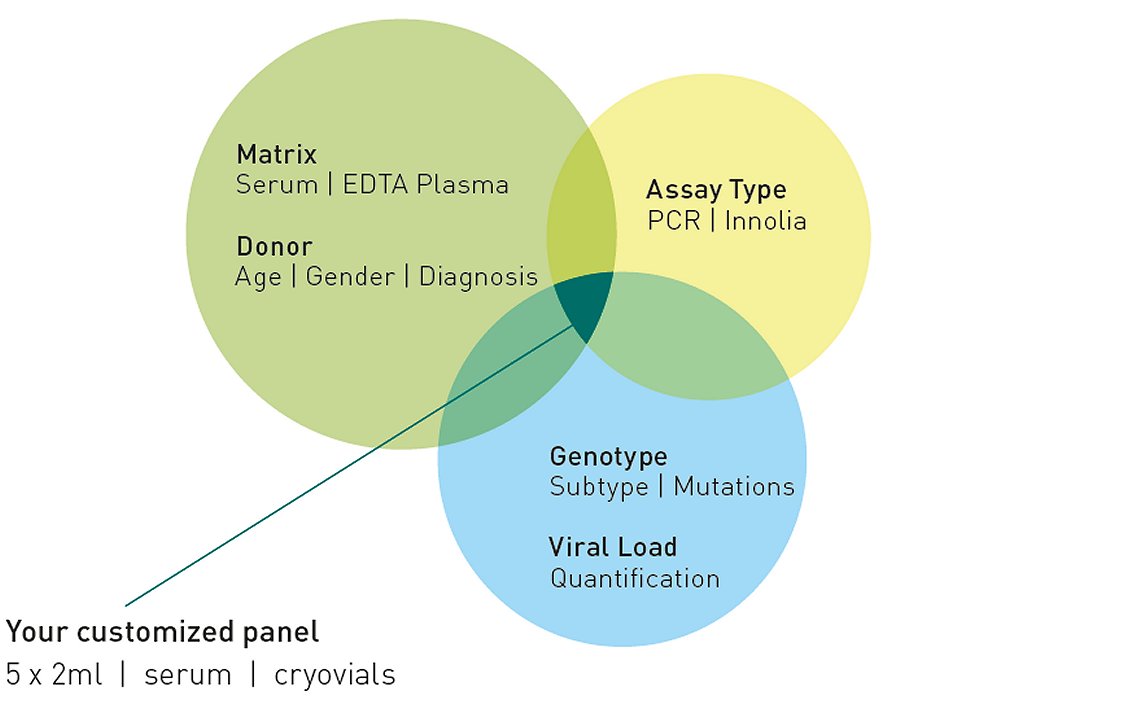
The full list of products is available, please contact us.
R&D | assay validation
TRINA Clinical Services
Large variety of biological products and services, backed by expertise in virology, serology, immunology and molecular biology.
Laboratory testing services, including parallel testing
• Donor Identification / screenings for Markers / Conditions
• Management and analysis of collected data
• Sample management and logistical support
Clinical trial support
• Customized clinical data
Assay Validation
• Performance validation FDA / CE
• Screenings
• Quality monitoring materials
pre-clinical specimen | patient samples
TRINA BIOREACTIVES provides biological material for research in diagnostic and pharmaceutical industries. Collected specimen are fully compliant with actual ethical and regulatory criteria (IRB approval / ICF documentation).
| Clinical Specimen |
| Over 50’000 donors for biospecimen from globally different diagnostic and demographic individuals. (Europe,
Asia, Americas, Africa) Different Matrixes (Serum, EDTA, LiHep, etc.) & Multibleeds 1 - 800 ml per Donor. Clinical Data: Patient Data (Age / Gender / GT). Detailed Anamnesis (prospective). |
| Isolated Cell Lines / PBMC& |
| Customized, cryopreserved, ready to use isolated cell lines from specific selected donors. (Donor characteristics such as: Allergic / PMN enriched / vaccination boosted). The isolation protocol is designed to meet your assay's specifications. www.trinacell.com |
| Assay Validation & Pre-clinical |
| Technical performance validation for chemiluminescence, point of care and molecular based IVD applications: A large donor base (> 5000 Donors in any serum / plasma matrix) for positive / negative / quantifications & validations. |
